Best known for setting up and flipping WA and South Australia's IKEA stores back to its parent company in 2017, Alan Tribe's PYC Therapeutics is raising $146 million to conduct human trials of up to four of its clinical-stage biotech assets.
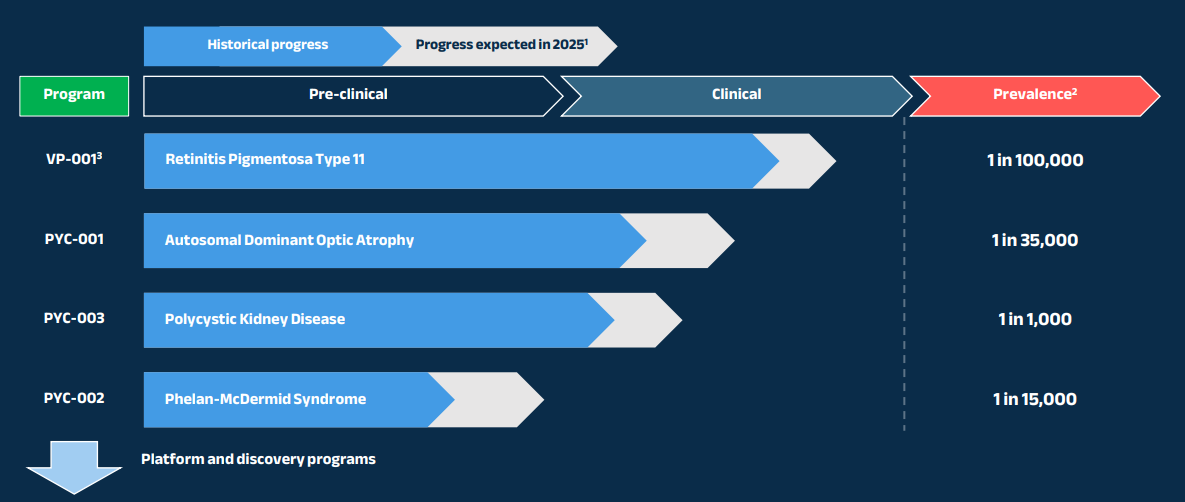
The biotech company - which has been listed on the ASX for two decades - is set to release a pipeline of groundbreaking ribonucleic acid (RNA) based drugs including candidates PYC-001 to PYC-003 and VP-001 to treat monogenic blindness and lethal gene disorders that attack the kidneys.
Cap raise
Funds for the ~$600 million market-capped drug inventor will be raised through a 1 for 4 pro-rata accelerated non-renounceable entitlement offer at $1.25/sh to raise ~$145.8 million - a 2.7% discount to the last trading price of $1.28 and made available to both institutional and retail investors in Australia and New Zealand.
Putting further skin in the game, PYC Therapeutics (ASX : PYC) chairman Alan Tribe has subscribed to $35 million worth of new shares under the entitlement offer, representing ~34% of PYC’s issued capital.
Proceeds from the equity raising will add to >$200 million ready to fund the progression of the company’s pipeline of first-in-class drug candidates for patients with major unmet needs through critical human data read-outs.
With capital in place, all four pipeline programs are expected to be fully funded through FY27.
Pipeline of RNA drug trials
Proceeds from the cap raise will be used to:
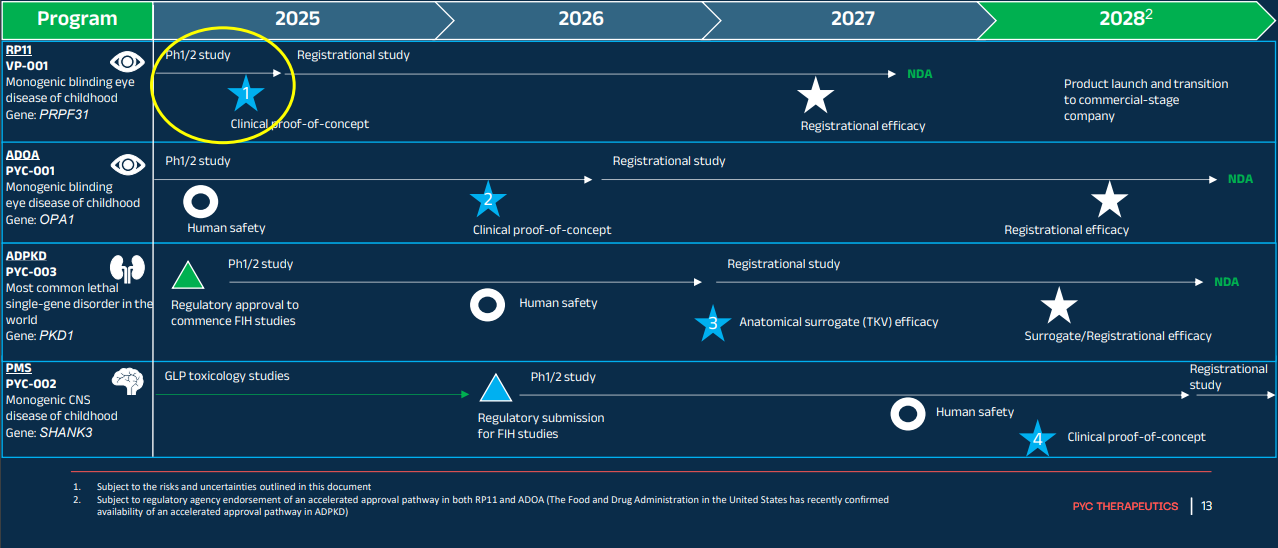
- Progress a first blinding eye disease drug candidate into late-stage human trials (VP-001)
- Progress a second blinding eye disease drug candidate into a mid-stage human trial (PYC-001)
- Progress a polycystic kidney disease drug candidate through early-stage human trials (PYC-002)
- Support progression of the Phelan-McDermid Syndrome (PMS) drug program into human trials (PYC-003)
- Further drug discovery and platform development efforts
The funding extension will deliver human safety and efficacy data in four drug programs, with clinical efficacy data for the first drug candidate to progress into human trials in Retinitis Pigmentosa 11 (with VP-001) due in Q2 2025.
PYC has already observed a proof of concept for VP-001:
“[My patient] now sees airplanes in the sky (never had before), stars at night, animals/creatures along the road … the stories
go on” - Principal Investigator in PYC’s RP type 11 phase 1/2 clinical trial
Other milestones the company aims to hit this year include:
- Collating safety and efficacy data for all patients enrolled in the ongoing Phase 1 & 2 studies
- FDA confirmation of pathway to a New Drug Application
- Commencement of a registration trial
- Continuation of the Phase 1 & 2 studies in an open-label format to demonstrate long-term efficacy in patients >12 months