Healthy profit-taking may explain the 5% drop in Mesoblast’s (ASX: MSB) share price today, but the dual-listed ASX/NASDAQ biotech stock is still up over 20% since winning FDA approval for its Ryoncil drug on 19 December.
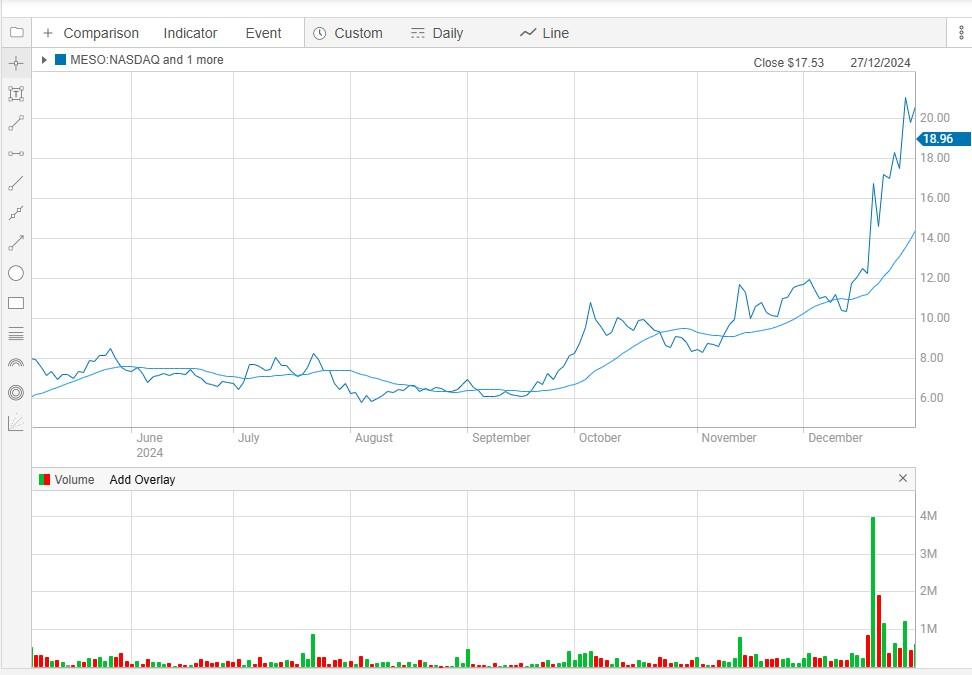
While the company is no stranger to good news, a spike in recent trading volumes suggests the stock is currently enjoying a strong bullish trend (see table). This marks a noteworthy spike given that the share price is up around 1000% since trading at $0.29 early December 2024.
Despite receiving little to no (key) broker coverage, the biotech darling is popular with retail investors and some buying activity also reflects its size. A market cap of $3.2 billion puts Mesoblast well within ASX200 — which means certain funds are required to buy it.
What’s also attracting global institutional investors to the stock was its inclusion onto the Nasdaq Biotechnology Index last year.
First under the wire
Revelations late last month that its Ryoncil drug was the first approval for any kind of mesenchymal stromal cell therapy in the US - for any indication – was a watershed moment for the stock.
The company’s CEO Silviu Itescu believes the significance of being able to start selling Ryoncil, almost immediately, should not be underestimated.
The drug Ryoncil is currently approved to treat children who suffer adverse effects after allogeneic bone marrow transplants.
There’s now potential now to expand the treatment into the adult cohort, and Itescu also expects the “halo effect” from Ryoncil’s FDA approval to help get future products over the line.
“This approval should provide the confidence that shareholders are looking for in terms of the likelihood of multiple product approvals as we move forward,” said Itescu.
Meanwhile, revenue from Ryoncil is expected to help fund the company’s other drug candidates – Revascor for cardiovascular disease and rexlemestrocel-L for inflammatory pain conditions.
Second generational platform
“There’s a flow-on effect to the rest of our pipeline… our second generation platform, rexlemestrocel, is being developed for cardiovascular disease, for inflammatory back pain – multibillion-dollar opportunities, much larger than GVHD, and those are either in phase three or have completed phase three,” said Itescu.
“So those are the next ones that we will aim to get in front of the FDA and potentially over the line next year and beyond that.”
While Mesoblast delivered an earnings loss of -$56.2 million in FY24, greater than forecast, it has has provided no guidance on future earnings.